Water is one of the most essential substances on Earth, covering about 71% of the planet’s surface and serving as a vital resource for all living organisms. One of its most fascinating properties is its transparency. Unlike many other substances that appear opaque or colored, water allows light to pass through, making it clear to the human eye. But what exactly causes this unique characteristic?
Minimal Light Absorption
The primary reason water appears transparent is that it absorbs very little visible light. Light is composed of different wavelengths, which correspond to different colors. When light encounters a substance, it can be absorbed, reflected, or transmitted. Many materials absorb specific wavelengths while reflecting others, giving them distinct colors. However, water does not significantly absorb light in the visible spectrum (approximately 400–700 nanometers). Instead, it absorbs more in the infrared and ultraviolet ranges, which are not visible to the human eye.
Limited Scattering of Light
Scattering occurs when light waves bounce off particles or irregularities within a substance. In opaque materials, scattering is extensive because the internal structure disrupts the passage of light. In contrast, pure water has a uniform molecular structure with very small particles that do not significantly scatter visible light. This allows light to pass through easily, maintaining water’s transparent appearance.
Molecular Structure of Water
Water is composed of two hydrogen atoms and one oxygen atom (H₂O), forming a simple yet highly stable molecular structure. Unlike crystalline solids or cloudy liquids that have complex arrangements causing light to diffuse, water molecules are loosely bonded in a way that does not obstruct the transmission of light. This molecular arrangement enables light to pass through with minimal interference.
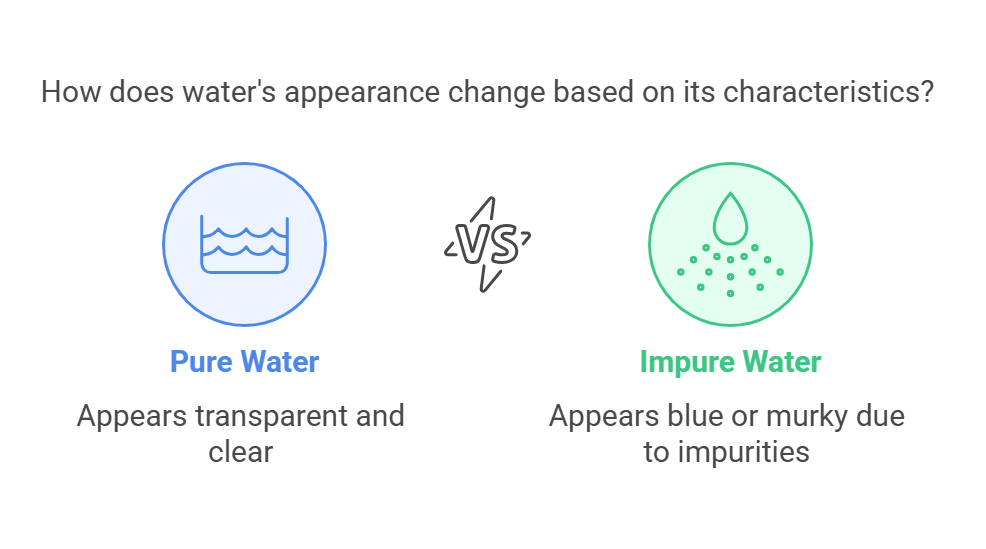
Effects of Depth and Impurities
While pure water is transparent, its appearance can change depending on depth and the presence of impurities. In large bodies of water, such as oceans or lakes, water often appears blue because it absorbs longer wavelengths of light (such as red and yellow) more efficiently than shorter wavelengths (like blue and violet). Additionally, suspended particles, dissolved substances, or microorganisms can scatter and absorb light, making water appear murky or colored.
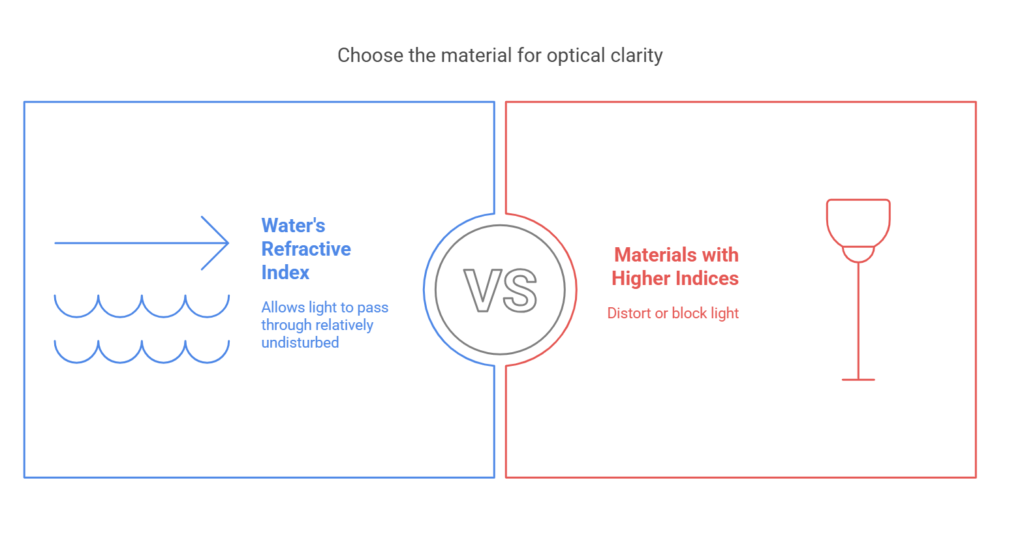
The Role of Refraction
Another factor contributing to water’s transparency is its refractive index, which is about 1.33. This means that water bends light slightly when it enters but does not significantly disrupt its passage. Unlike materials with higher refractive indices that distort or block light, water allows light to pass through relatively undisturbed, reinforcing its transparent nature.
Conclusion
Water’s transparency is a result of its minimal absorption of visible light, limited scattering, and simple molecular structure. While factors like depth and impurities can influence its appearance, pure water remains clear due to its ability to transmit light efficiently. This property not only makes it visually unique but also plays a crucial role in supporting life by allowing sunlight to penetrate aquatic environments, enabling photosynthesis and sustaining ecosystems. Understanding why water is transparent helps us appreciate its remarkable physical characteristics and its significance in the natural world.










